22+ nernst equation calculator
The Nernst equation is an equation that relates the reduction potential of. In the input field enter the standard half-cell reduction potential chemical activity for the reductant and oxidant.
Nernst Equation Calculator Calculate Equilibrium Potential
The critical distance of a FRET process is the distance at which the energy transfer has 50 efficiency half of the energy of the donors relaxation is used by the acceptor.

. If oxygen is to be measured using a zirconia ceramic electrolyte and atmospheric air with pO 2 0209 as a reference this equation simplifies to. The Nernst equation calculates the equilibrium potential also referred to as the Nernst potential for an ion based on the charge on the ion ie its valence and its. All it takes is making sure that the coefficient of the highest power x² is one.
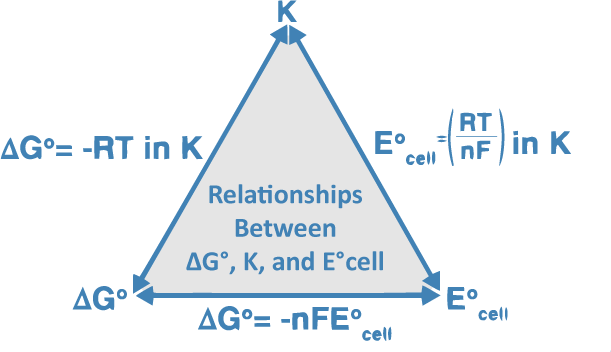
The equation was introduced by a. In electrochemistry the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction half-cell or full cell reaction from the. The Nernst equation can be used to calculate the equilibrium potential for an ion based on its charge valence and its concentration on each side of a membrane.
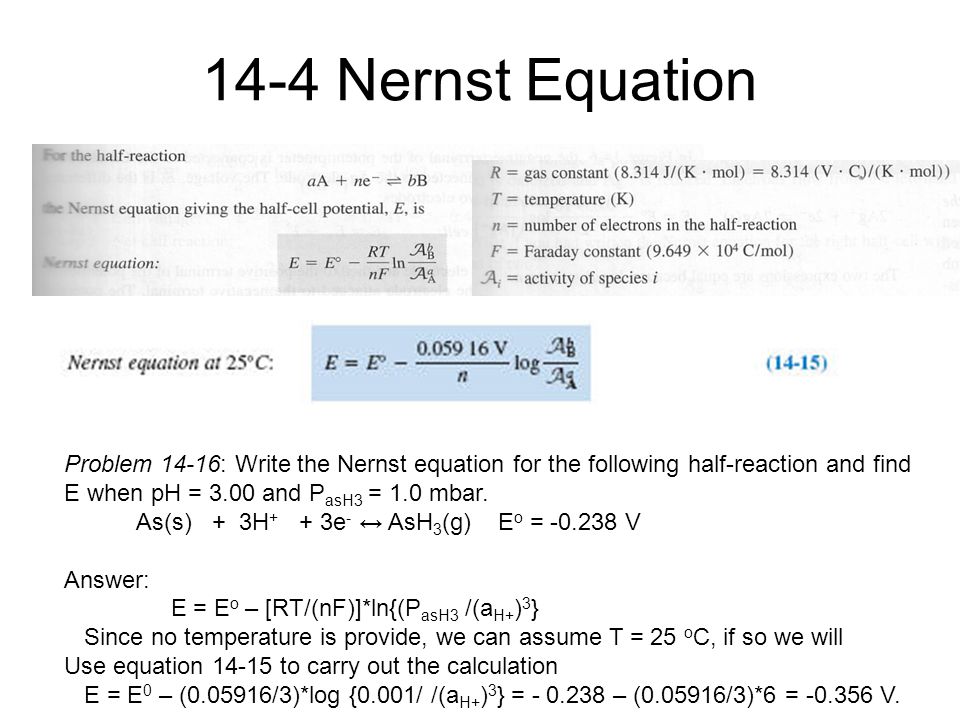
The nernst equation is illustrated by the image below. To compute for nernst equation seven essential parameters are needed and these parameters are Electrochemical. E E0 00592n log_10 Q Hence as per the Nernst equation the potential of the electrochemical cell depends on the.
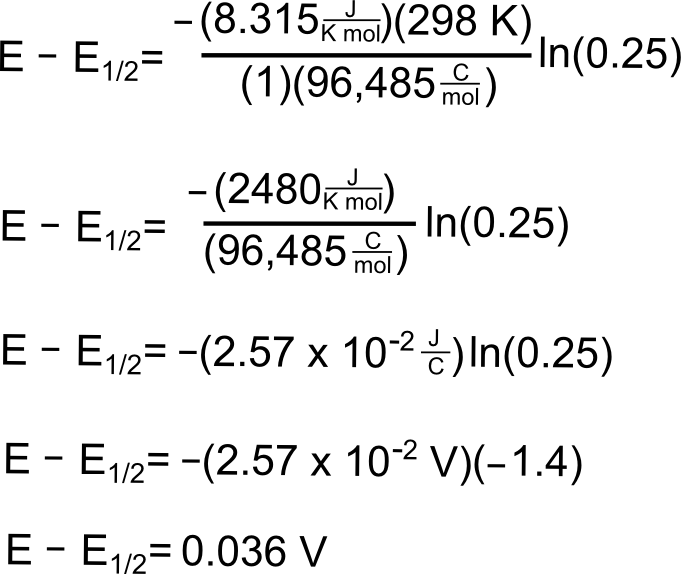
PO 2 0209 exp-46421 ET The equation. The following example shows how the Nernst equation may be used to calculate the potential of an electrochemical cell at non-standard conditions. In the equation below Q is the reaction quotient and is.
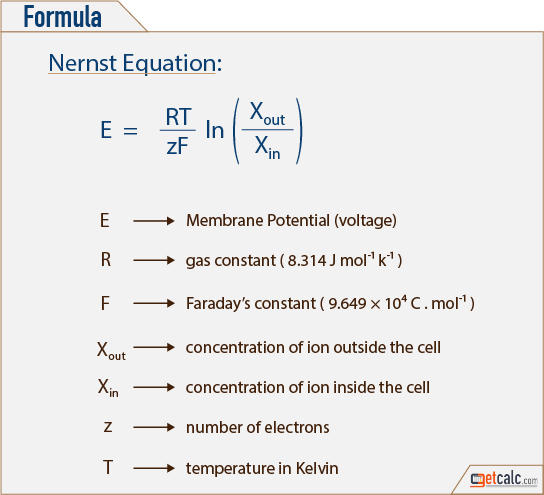
The Nernst equation for 298 Kelvin can be represented as follows. Tutor Pace offer students help with Nernst Equation Calculator for any grade in any subject including math algebra trigonometry and geometry. The Nernst equation describes the relationship between electrode potential and solution concentration.
The following is the procedure how to use the Nernst equation calculator Step 1. However in non-standard conditions the Nernst equation is used to. Nernst equation calculator for converting between oxygen and voltage at a set temperature.
Standard cell potentials are calculated in standard conditions of temperature and pressure. This voltage is often. The Nernst equation is often used to calculate the cell potential of an electrochemical cell at any given temperature pressure and reactant concentration.
Easy is good so we basically want to force the quadratic equation into the form xa²x²2axa².
Nernst Equation Calculator Calistry
Nernst Equation Calculator
The Nernst Equation

Nernst Equation Calculator Calculate Equilibrium Potential
Chemistry Analytical Chemistry Fall Ppt Video Online Download
Nernst Equation Calculator Calistry

Calculating Cell Potential Using The Nernst Equation Youtube

Nernst Equation Wikipedia
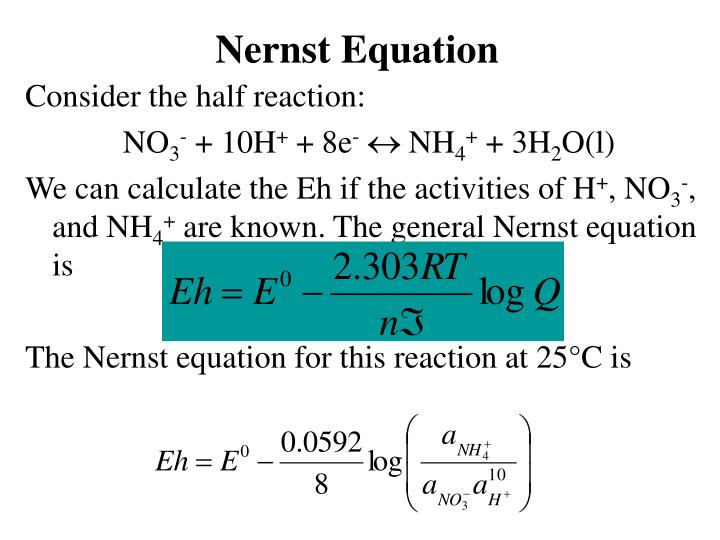
Ppt Nernst Equation Powerpoint Presentation Free Download Id 3432521

Nernst Equation Video Khan Academy
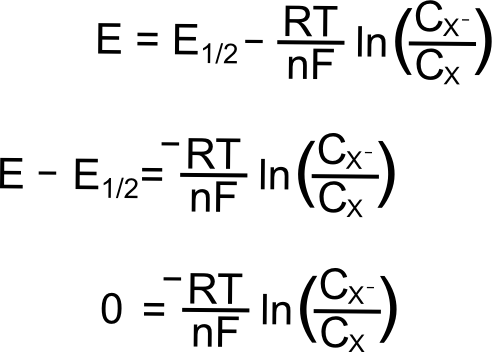
The Nernst Equation
Rmp Theory Nernst Equation
Nernst Equation Calculator Calculator Academy

Nernst Equation An Overview Sciencedirect Topics

Nernst Equation Calculator Calculate Equilibrium Potential
Nernst 7

Concentration Cell Direction Of Electron Flow Nernst Equation Electrochemistry Mcat Content